Why do some men not produce sperm? Stowers scientists collaborate to uncover one underlying reason for male infertility
KANSAS CITY, Mo., Oct. 20, 2023 /PRNewswire/ — Millions of couples worldwide experience infertility with half of the cases originating in men. For 10% of infertile males, little or no sperm are produced. Now, new research from the Stowers Institute for Medical Research, in collaboration with the Wellcome Centre for Cell Biology at the University of Edinburgh, is shedding light on what may be going wrong in the process of sperm formation, leading to potential theories on possible treatments.
If you know exactly what is wrong, there are technologies emerging right now that might give you a way to fix it
Stowers scientists collaborate to uncover one underlying reason for male infertility. Millions of couples worldwide experience infertility with half of the cases originating in men. For 10% of infertile males, little or no sperm are produced. Now, new research from the Stowers Institute for Medical Research, in collaboration with the Wellcome Centre for Cell Biology at the University of Edinburgh, is shedding light on what may be going wrong in the process of sperm formation, leading to potenti
Microscopy images showing normal seminiferous tubules in control testes with mature sperm (black arrow: left) but smaller empty seminiferous tubules in testes harboring a synaptonemal complex protein point mutation (black asterisk: right).
Representative testes from 9-week-old control mice (left) and mice with a point mutation in one synaptonemal complex protein (right).
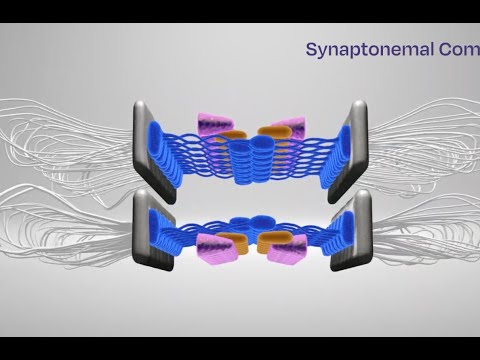
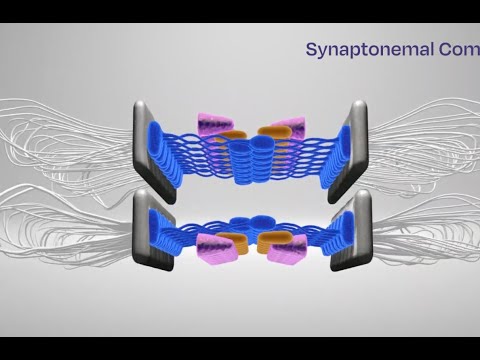
Model of the synaptonemal complex in control and mutant mice. The protein the team investigated (SYCP1) forms normally, and all additional necessary proteins are recruited. In the mutant, SYCP1 localizes to the chromosome axes but does not successfully form the bridge-like structure (head-to-head interactions), and the additional proteins that help keep the bridge intact are either missing or not properly organized.
“A significant cause of infertility in males is that they just cannot make sperm,” said Stowers Investigator Scott Hawley, Ph.D. “If you know exactly what is wrong, there are technologies emerging right now that might give you a way to fix it.”
The study published on October 20, 2023, in Science Advances from the Hawley Lab and Wellcome Centre Investigator Owen Davies, Ph.D., may help explain why some men do not make enough sperm to fertilize an egg. In most sexually reproducing species, including humans, a critical protein structure resembling a lattice-like bridge needs to be built properly to produce sperm and egg cells. The team led by former Postdoctoral Research Associate Katherine Billmyre, Ph.D., discovered that in mice, changing a single and very specific point in this bridge caused it to collapse, leading to infertility and thus providing insight into human infertility in males due to similar problems with meiosis.
Meiosis, the cell division process giving rise to sperm and eggs, involves several steps, one of which is the formation of a large protein structure called the synaptonemal complex. Like a bridge, the complex holds chromosome pairs in place enabling necessary genetic exchanges to occur that are essential for the chromosomes to then correctly separate into sperm and eggs.
“A significant contributor to infertility is defects in meiosis,” said Billmyre. “To understand how chromosomes separate into reproductive cells correctly, we are really interested in what happens right before that when the synaptonemal complex forms between them.”
Previous studies have examined many proteins comprising the synaptonemal complex, how they interact with each other, and have identified various mutations linked to male infertility. The protein the researchers investigated in this study forms the lattices of the proverbial bridge, which has a section found in humans, mice, and most other vertebrates suggesting it is critical for assembly. Modeling different mutations in a potentially crucial region in the human protein enabled the team to predict which of these might disrupt protein function.
The authors used a precise gene editing technique to make mutations in one key synaptonemal complex protein in mice, which allowed the researchers, for the first time, to test the function of key regions of the protein in live animals. Just a single mutation, predicted from the modeling experiments, was verified as the culprit of infertility in mice.
“We’re talking about pinpoint surgery here,” said Hawley. “We focused on a tiny little region of one protein in this gigantic structure that we were pretty sure could be a significant cause of infertility.”
Mice have long been used as models for human diseases. From the modeling experiments using human protein sequences, along with the high conservation of this protein structure across species, the precise molecule that caused infertility in mice likely functions the same way in humans.
“What is really exciting to me is that our research can help us understand this really basic process that is necessary for life,” said Billmyre.
For Hawley, this research is a true representation of the versatility of the Institute. Hawley’s lab typically conducts research in fruit flies, yet the protein discovered in this study was not present in fruit flies and demanded a different research organism to continue. Because of the resources and Technology Centers at the Institute, it was possible to quickly pivot and test the new infertility hypothesis in mice.
“I can’t imagine another place where this could happen,” said Hawley. “I think it’s an amazing example of how the Stowers Institute’s dedication toward discovery can yield big results providing important leaps forward in understanding.”
Additional authors include Emily A. Kesler, Dai Tsuchiya, Ph.D., Timothy J. Corbin, Kyle Weaver, Andrea Moran, Zulin Yu, Ph.D., Lane Adams, Kym Delventhal, Michael Durnin, Ph.D., and Owen Richard Davies, Ph.D.
This work was funded by the Wellcome Centre for Cell Biology (award: 203149), the Wellcome Senior Research Fellowship (award: 219413/Z/19/Z), and by institutional support from the Stowers Institute for Medical Research.
About the Stowers Institute for Medical Research
Founded in 1994 through the generosity of Jim Stowers, founder of American Century Investments, and his wife, Virginia, the Stowers Institute for Medical Research is a non-profit, biomedical research organization with a focus on foundational research. Its mission is to expand our understanding of the secrets of life and improve life’s quality through innovative approaches to the causes, treatment, and prevention of diseases.
The Institute consists of 20 independent research programs. Of the approximately 500 members, over 370 are scientific staff that include principal investigators, technology center directors, postdoctoral scientists, graduate students, and technical support staff. Learn more about the Institute at www.stowers.org and about its graduate program at www.stowers.org/gradschool.
Media Contact:Joe Chiodo, Head of Media Relations
724.462.8529
[email protected]
SOURCE Stowers Institute for Medical Research

Also from this source

PRN Top Stories Newsletters
Sign up to get PRN’s top stories and curated news delivered to your inbox weekly!
Thank you for subscribing!

By signing up you agree to receive content from us.Our newsletters contain tracking pixels to help us deliver unique content based on each subscriber’s engagement and interests. For more information on how we will use your data to ensure we send you relevant content please visit our PRN Consumer Newsletter Privacy Notice. You can withdraw your consent at any time in the footer of every email you’ll receive. Mit Ihrer Anmeldung erklären Sie sich damit einverstanden, Inhalte von uns zu erhalten.Unsere Newsletter enthalten Zählpixel, die die Lieferung einzigartiger Inhalte in Bezug auf das Abonnement und die Interessen der einzelnen Abonnenten ermöglichen. Weitere Informationen über die Verwendung Ihrer Daten im Hinblick auf die Zusendung von relevanten Inhalten, finden Sie in unserer PRN Consumer Newsletter Privacy Notice. Ihre Zustimmung können Sie jederzeit in der Fußzeile jeder erhaltenen E-Mail widerrufen. En vous inscrivant à la newsletter, vous consentez à la réception de contenus de notre part.Notre newsletter contient des pixels espions nous permettant la fourniture à chaque abonné, d’un contenu unique en lien avec ses souscriptions et intérêts. Pour de plus amples informations sur l’utilisation faite de vos données en vue de l’envoi des contenus concernés, nous vous invitons à consulter la politique de confidentialité disponible à partir du lien suivant PRN Consumer Newsletter Privacy Notice. Vous pouvez à tout moment revenir sur votre consentement par le biais des informations situées au bas de chaque e-mail reçu. Регистрирайки се, Вие се съгласявате да получавате информационно съдържание от нас. Нашите бюлетини съдържат проследяващи пиксели, които ни помагат да предоставяме уникално съдържание въз основа на ангажираността и интересите на всеки абонат. За повече информация относно начина, по който ще използваме Вашите данни, за да гарантираме, че Ви изпращаме подходящо съдържание, моля, направете справка с нашето Уведомление за поверителност на потребителския бюлетин на PRN. Можете да оттеглите съгласието си по всяко време в долния колонтитул на всеки от имейлите, които ще получите.